Lithium battery knowledge-what is lithium battery?
Lithium batteries have been closely related to our lives,our food,clothing,housing and transportation are inextricably linked with it.However,many people do not understand lithium batteries.This is not limited to ordinary consumers.Even the designers of various electrical appliances have little understanding of lithium batteries.A series of articles on lithium battery knowledge will be shared below.Through these articles,you will have a systematic understanding of lithium batteries.
The lithium-ion batteries mentioned here specifically refer to secondary lithium-ion batteries that can be recharged over and over again,rather than primary batteries that are thrown away after use.Lithium-ion batteries are distributed in all corners of our lives.Their applications include cell phones,tablet computers,laptops,smart watches,mobile power(rechargeable treasure),emergency power,shavers,electric bicycles,electric cars,electric buses,tour buses,drones and other electric tools.As a carrier of electricity and a source of power for many devices,it's safe to say that today's material world would be unplayable without lithium-ion batteries(unless we want to go back a few decades).So,what is a lithium-ion battery?
This article does not popularize the basic principles and development history of the battery.Baidu if you are interested.There are a lot of stories here.Fundamental theories in physics and chemistry were largely confused by the wave before Einstein.Batteries are directly related to these two fields.Almost all of the theories related to batteries were researched before World War II,and there hasn't been much innovation since then.Theoretical research on lithium-ion batteries,a type of battery technology,has not made any breakthroughs in recent years.Most of the research has focused on materials,formulations,processes,etc.,i.e.,how to improve industrialization and develop lithium-ion batteries with better performance(more energy storage and longer range).
How to choose the energy carrier
First of all,you may ask,why choose lithium as the energy carrier?Well,although we don't want to review chemistry,we'll have to go to the periodic table to find the answer to this question.Luckily,everyone remembers the periodic table,right?I really don't remember,so let's take a moment to look at the table below.
If you want to be a good energy carrier,you have to store and carry more energy in the smallest possible size and weight.Therefore,the following basic conditions need to be met:
1)Small relative mass of atoms
2)High electron gain/loss capability
3)The ratio of electron transfers should be high
Based on these three fundamentals,elements at the top of the periodic table are better than those at the bottom,and elements on the left are better than those on the right.In a preliminary screening,we can only find materials from the first and second cycles of the periodic table:hydrogen,helium,lithium,beryllium,boron,carbon,nitrogen,oxygen,fluorine,and neon.Removing the noble gases and oxidizers leaves only hydrogen,lithium,beryllium,boron,and carbon.
Hydrogen is nature's best energy carrier,so research on hydrogen fuel cells has been booming,representing a very promising direction in the battery field.Of course,if nuclear fission technology can make a major breakthrough in the next few decades,and can be miniaturized or even miniaturized,then portable nuclear fuel cells will have a broad space for development.
The next step is lithium.The choice of lithium as a battery is based on the relatively optimal solution we can find among all the elements currently on the planet(beryllium's reserves are too small and it is the rarest of rare metals).The battle between hydrogen fuel cells and lithium-ion batteries as a technological route is in full swing in the field of electric vehicles,probably because these two elements are the better energy carriers we can find at the moment.Of course,there are many commercial interests and even political games involved.These are not the areas to be discussed in this article.
Incidentally,the energy sources that already exist in nature and are widely utilized by mankind,such as oil,natural gas,coal,etc.,are also mainly composed of the elements of carbon,hydrogen and oxygen(cycles of the first and second periodic tables).Therefore,whether it is natural selection or man-made"design",will eventually go the same way.
How lithium-ion batteries work
Let's start with the working mechanism of lithium-ion batteries.We will not describe redox reactions here.People with a bad chemistry foundation,or people who return their chemistry knowledge to their teachers,will feel dizzy when they see these specialized things,so we should describe them straightforwardly.Here to borrow a diagram,which is easier for people to understand the principle of lithium-ion battery.
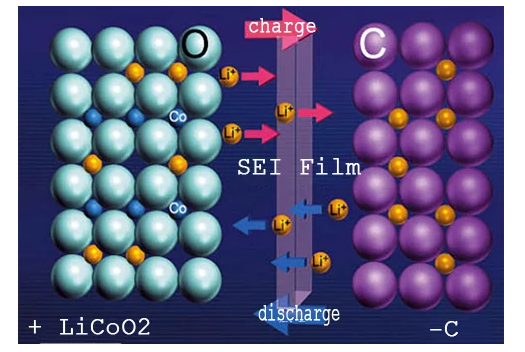
According to the habit of use,we distinguish the positive pole(+)and negative pole(-)according to the voltage difference during charging and discharging.The positive and negative terminals are not mentioned here,which is time-consuming and laborious.In this diagram,the positive material of the battery is lithium cobaltate(LiCoO2)and the negative material is graphite(C).
When charging,under the influence of an external electric field,the lithium element in the molecule of the positive material LiCoO2 detaches and becomes a positively charged lithium ion(Li+).Under the action of electric field force,it moves from the positive electrode to the negative electrode,and reacts chemically with the carbon atoms at the negative electrode to form LiC6,so the lithium ion running out from the positive electrode is very"stable"."Embedded in the graphite layered structure of the negative electrode.The more lithium ions that are run out of the positive electrode and transferred to the negative electrode,the more energy that battery can store.
The opposite is true when discharging.The internal electric field shifts and the lithium ion(Li+)detaches from the negative electrode.Following the direction of the electric field,it runs back to the positive electrode and becomes a lithium cobaltate molecule(LiCoO2)again.The more lithium ions that run out of the negative electrode and are transferred to the positive electrode,the more energy the battery releases.
In each charge/discharge cycle,lithium ions(Li+)as a carrier of electrical energy,over and over again from the positive electrode to the negative electrode and back to the positive electrode,and the positive and negative electrode material chemical reaction.In the process,the lithium ion(Li+)acts as a carrier of electrical energy from the positive electrode to the negative electrode and back to the positive electrode,reacting with the positive and negative electrode materials.This is the basic principle of"lithium ion battery".Because the electrolyte,isolation film,etc.are electronic insulator,so in this cycle,no electron back and forth movement between the positive and negative electrodes,only participate in the chemical reaction of the electrode.
Basic Composition of Lithium-ion Battery
In order to realize the above functions,lithium-ion batteries need to contain several basic materials inside:positive electrode active material,negative electrode active material,isolation film,and electrolyte.Let's briefly discuss what these materials are for.
Understanding the positive and negative electrodes is not difficult.In order to realize charge movement,positive and negative materials with potential difference are needed.So what is the active material?We know that batteries actually convert electrical and chemical energy into each other,thus enabling the storage and release of energy.To realize this process,it is necessary that the positive and negative materials are"easy"to participate in the chemical reaction,active,easy to oxidize and reduce,so as to realize the energy conversion,so it is necessary to use"active substances"as the positive and negative electrodes of the battery.
As mentioned above,lithium is our preferred battery material,so why not use lithium metal as the active substance for the electrodes?Isn't that the maximum energy density that can be achieved?
Let's look at the picture above again.The three elements of oxygen(O),cobalt(Co)and lithium(Li)form a very stable structure of the positive electrode material(the ratio and arrangement in the picture are for reference only),and the carbon atom arrangement of the negative electrode graphite also has a very stable layer structure.Positive and negative electrode materials must not only be active,but also have a very stable structure in order to achieve an orderly and controlled chemical reaction.What is the result of instability?Think of the burning of gasoline and the explosion of a bomb and the violent release of energy.The process of chemical reactions is practically impossible to control with human precision,so chemical energy becomes heat and energy is released all at once and irreversibly.
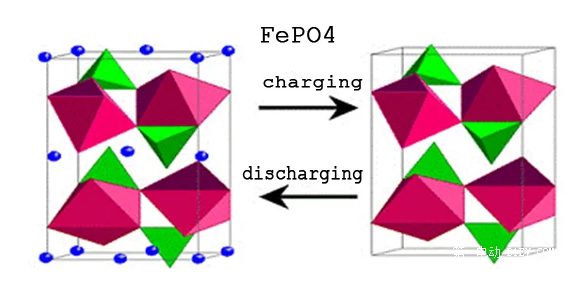
The metallic form of lithium is so"active"that most naughty children do not listen and like to destroy it.Early lithium battery research did focus on lithium metal or its alloys as the negative electrode,but due to prominent safety issues,in recent years,with the pursuit of energy density,this research direction has a"blood resurrection"trend,we will talk about later.
In order to achieve chemical stability during energy storage and release,i.e.,the safety and long life of the battery charge/discharge cycle,we need an electrode material that is active when it is active and stable when it is active.Maintain stability.After a long period of research and exploration,people have discovered several metal oxides of lithium,such as lithium cobaltate,lithium titanate,lithium iron phosphate,lithium manganate,nickel-cobalt-manganese ternary and other materials,as the active material of lithium.As shown in the figure above,the olivine structure of lithium iron phosphate is also a very stable structure of anode material.The de-embedding of lithium ions during charging and discharging does not lead to lattice collapse.Off-topic,lithium metal batteries do have,but compared with lithium-ion batteries,it is almost negligible.The development of technology should ultimately serve the market.
Of course,in solving the stability problem,but also brought a serious"side effect".That is,the proportion of lithium as an energy carrier is greatly reduced,the energy density is reduced by more than one order of magnitude.Gains and losses,the natural way.
Graphite or other carbon materials are often used as the active material of the negative electrode.They also follow the above principles.Not only do they need to be good energy carriers,but they also need to be relatively stable,relatively abundant in reserves,and easy to manufacture on a large scale.Looking around,carbon is a relatively optimal solution.Of course,it is not the only solution.There is extensive research on negative electrode materials,which will be discussed later.
What is the role of electrolytes?In layman's terms,it is the"water"in the swimming pool that allows the lithium ions to swim freely.Therefore,the ionic conductivity should be high(swimming resistance is small),the electronic conductivity should be small(insulation),chemical stability should be good(stability is overwhelming),thermal stability should be good(all for the sake of safety)based on these principles,after a long period of engineering exploration,people have found by the high purity of organic solvents,electrolyte lithium salts and the necessary additives made of electrolyte.The electrolyte is formulated under certain conditions and in certain proportions.Organic solvents include PC(propylene carbonate),EC(ethylene carbonate),DMC(dimethyl carbonate),DEC(diethyl carbonate),EMC(ethyl methyl carbonate)and other materials.Electrolyte lithium salts are LiPF6,LiBF4 and other materials.
The isolation film is added to prevent direct contact between the positive and negative electrode materials.We want to make the battery as small as possible and store as much energy as possible,so the distance between the positive and negative electrodes is getting smaller and smaller,and short-circuiting becomes a huge risk.In order to prevent the positive and negative materials from short-circuiting and resulting in a dramatic release of energy,a material is needed to"isolate"the positive and negative electrodes.This is the origin of the isolation film.Isolation membrane needs to have good ion permeability,mainly for lithium ion free passage to open the channel,and at the same time is an insulator of electrons,to realize the insulation between the positive and negative electrodes.Currently on the market,there are mainly single-layer PP,single-layer PE,double-layer PP/PE,three-layer PP/PE/PP composite membrane.
4.Complete material composition of lithium-ion battery
In addition to the above four main materials,in order to make lithium-ion batteries from the laboratory"experimental products"into commercially available products,but also need some other indispensable materials.
In addition to the active material,there are conductive agents and binders,as well as the substrate and the current collector(usually aluminum foil for the positive electrode)used as the current carrier.The binder should uniformly"fix"the lithium metal oxide as the active substance to the positive substrate,and the conductive agent should enhance the conductivity of both the active substance and the substrate in order to achieve a higher charging and discharging current.The collector is responsible for acting as a charge transfer bridge between the inside and outside of the battery.The structure of the negative electrode is essentially the same as that of the positive electrode.An adhesive is needed to hold the active substance graphite,a copper foil as a substrate and a collector as a conductor of current.
However,due to the good conductivity of graphite itself,the negative electrode generally does not add a conductor material.In addition to the above materials,a complete lithium-ion battery also includes insulating sheets,covers,pressure relief valves,shells(aluminum,steel,composite film,etc.)and other auxiliary materials.
Lithium-ion battery production process
Lithium-ion battery production process is more complex,here is only a brief introduction to some of the key processes.According to the assembly method of the pole piece,there are usually two process routes:winding and lamination.
The lamination process is to cut the positive and negative electrodes into small pieces and stack the isolation film to synthesize small cell monomers,and then the small cell monomers are stacked in parallel to form a large battery manufacturing process.The general process flow is as follows:
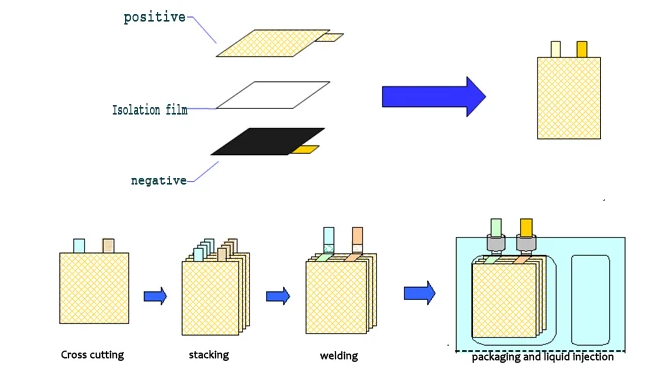
Winding process is to fix materials such as positive and negative pole pieces,isolation film,positive and negative pole lugs,protection tape,terminal tape,etc.on the equipment,and the equipment unwinds to complete the production of battery cells.
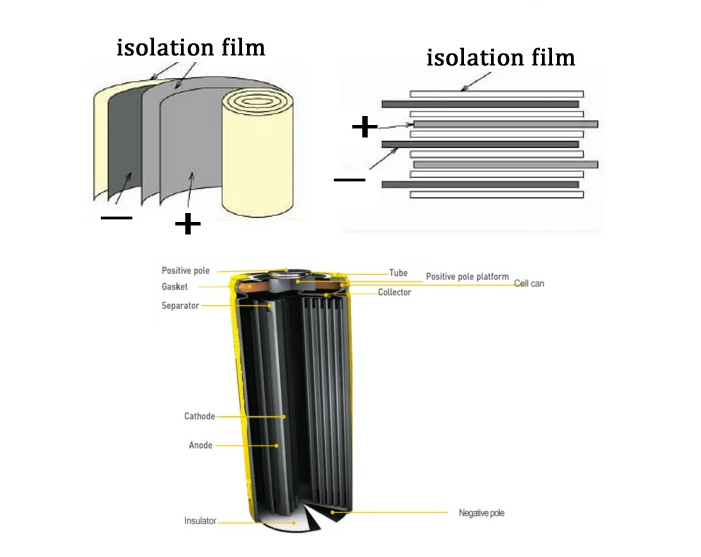
The common shapes of lithium ion batteries are mainly cylindrical and square.According to the different shell materials,there are metal shells and soft packaging shells.That's the end of our sharing today.We will provide more articles about lithium batteries.Stay tuned!

 简体中文
简体中文 Russian
Russian French
French German
German Japanese
Japanese Korean
Korean Arabic
Arabic Spanish
Spanish





